Brabender:
Mini-Compounder KETSE 12/36
The scale-down of the machine combined with the comprehensive adaptability to almost each processing task and the easy handling of the system constitute the distinction of the mini-compounder as an excellent laboratory instrument.
The processing unit of the mini-compounder consists of a horizontally divided barrel with a length of 36 D which can be tilted open and allows visual assessment of the processing steps. Thus, comfortable removal and quick cleaning of the barrel is allowed. In addition to the main metering, metering or venting ports at a barrel length of 6 D, 12 D and 26 D are available. Necessary side feeders can be installed at a processing length of 14 D.
Anton Paar Certified Service
- More than 350 manufacturer-certified technical experts worldwide
- Qualified support in your local language
- Protection for your investment throughout its lifecycle
- 3-year warranty
Documents
-
Brochure | Mini-Compounder KETSE 12/36 D Brochures
Accessories
Accessories
Accessories
Accessories
If you do not find the item you require, please contact your Anton Paar sales representative.
To find out if you can purchase online from your location, check the online availability below.

Brabender:
Blown Film Take-Off Unit
- User-friendly: Working height, winding torque and cooling air adjustable via touchscreen
- Application versatility: Adaptable to different film dimensions and properties
- Expandable: Measure and control film width and analyze film quality using optional modules
- MetaBridge Connect: Full control and automated data transfer to Brabender MetaBridge extruders

Brabender:
Conveyor Belt
- Adjustable height
- Continuously adjustable speed

Brabender:
CuttingDevice
- Easy integration: control and logging of knife speed via the software
- Compact: optimal dimensions for laboratory operation
- Flexibile: Knife holder extenable from 4 to 8 knifes, hence higher cutting frequency
- Hygienic design: Easy access to the parts to be cleaned
- Safety: Zero position of the knife does not stop directly in front of the die
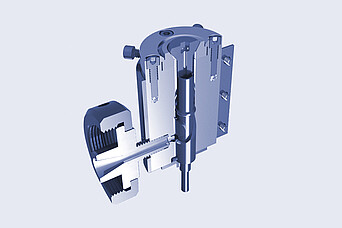
Brabender:
Film-Blowing Die Head
- spiral mandrel distributors
- uills with easily exchangeable inserts

Brabender:
Filtratest
- Quick change of sieves with drawer system
- Integrated pre-heating of the sieve package
- Short-time cycle times
- continuous extrusion procedure by bypass option of the melt flow
- Comfortable Windows Software
- Option: sliding funnel combination for flushing ring and sample
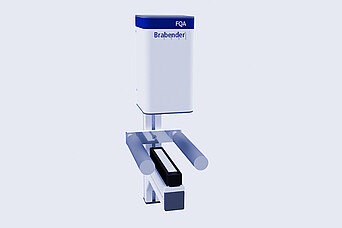
Brabender:
FQA Film Quality Analyzer
- Versatile: Identification of different defect types
- Efficient: Automatic evaluation according to size classes
- Continuous: Quality inspection in the running process
- Flexible: Suitable for laboratory systems and production use

Brabender:
Pasta Die Head
- can be cooled with both air and water
- for pasta and similar products

Brabender:
Pelletizer
- Uniform pelletizing
- Variable pellet sizes: stepless adjustment of positioning and outfeed speed
- Various pellet lengths
- Low-noise operation
- Simple loading
- Operated manually or via PC
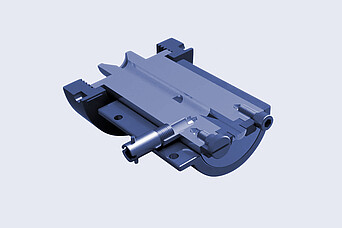
Brabender:
Rheometric Round Capillary Die Head
- Continuous operation
- Coverage of the occurring shear rate range
- Accurate viscosity and shear rate data with the corresponding corrections calculated by the software
- All acquired values are suitable to evaluate the shear and thermal stability of your material
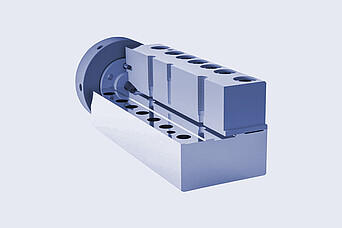
Brabender:
Rheometric Slot Capillary Die Head
- Continuous operation Coverage of the occurring shear rate range
- Accurate viscosity and shear rate data with the corresponding corrections calculated by the software
- All acquired values are suitable to evaluate the shear and thermal stability of your material Requests

Brabender:
Ribbon Die Head
- suitable for applications in plastics, rubber, food, and feed
- flexibility in terms of the belt thickness and width
- special geometries available on request
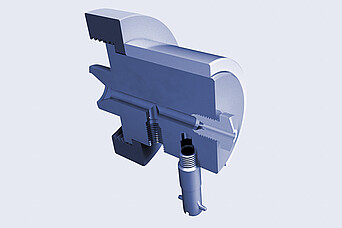
Brabender:
Round Strand Die Head
- equipped with a heating collar
- diameter of 1 mm to 7 mm
- can be equipped with a cutting device

Brabender:
Swelltest
- Calculation of the swell value
- High-precision, non-contact measurement
- Continuous diameter measurement
- With diagram printout
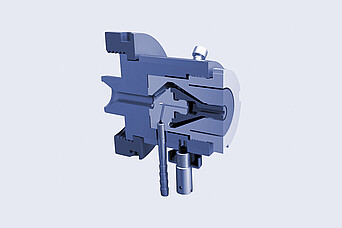
Brabender:
Tubing Die Head
- easily exchangeable die inserts
- for different diameters and material strengths

Brabender:
Univex
- Excellent film quality
- High take off speed
- Accurate temperature conditioning

Brabender:
Vertical Forced Feeder
- Small sample sizes
- Compact
- Easy to clean

Brabender:
Water Bath
- Adjustable height
- With overflow valve

Brabender:
Winder
- Flexible and easy retrofitting of roll to belt haul off or vice versa
- Supports for various types of coils
- Precise setting of the haul off speed
- Adjustable distance between haul off and oscillating unit
- Ergonomic control panel
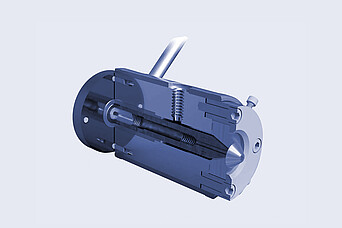
Brabender:
Wire Coating Die Head
- different diameters are possible
- wire production line on a laboratory scale
- Wrap wires with polymer coatings

Brabender:
Wire Take-Off Unit
- With Waterbath
- With CAN Bus
